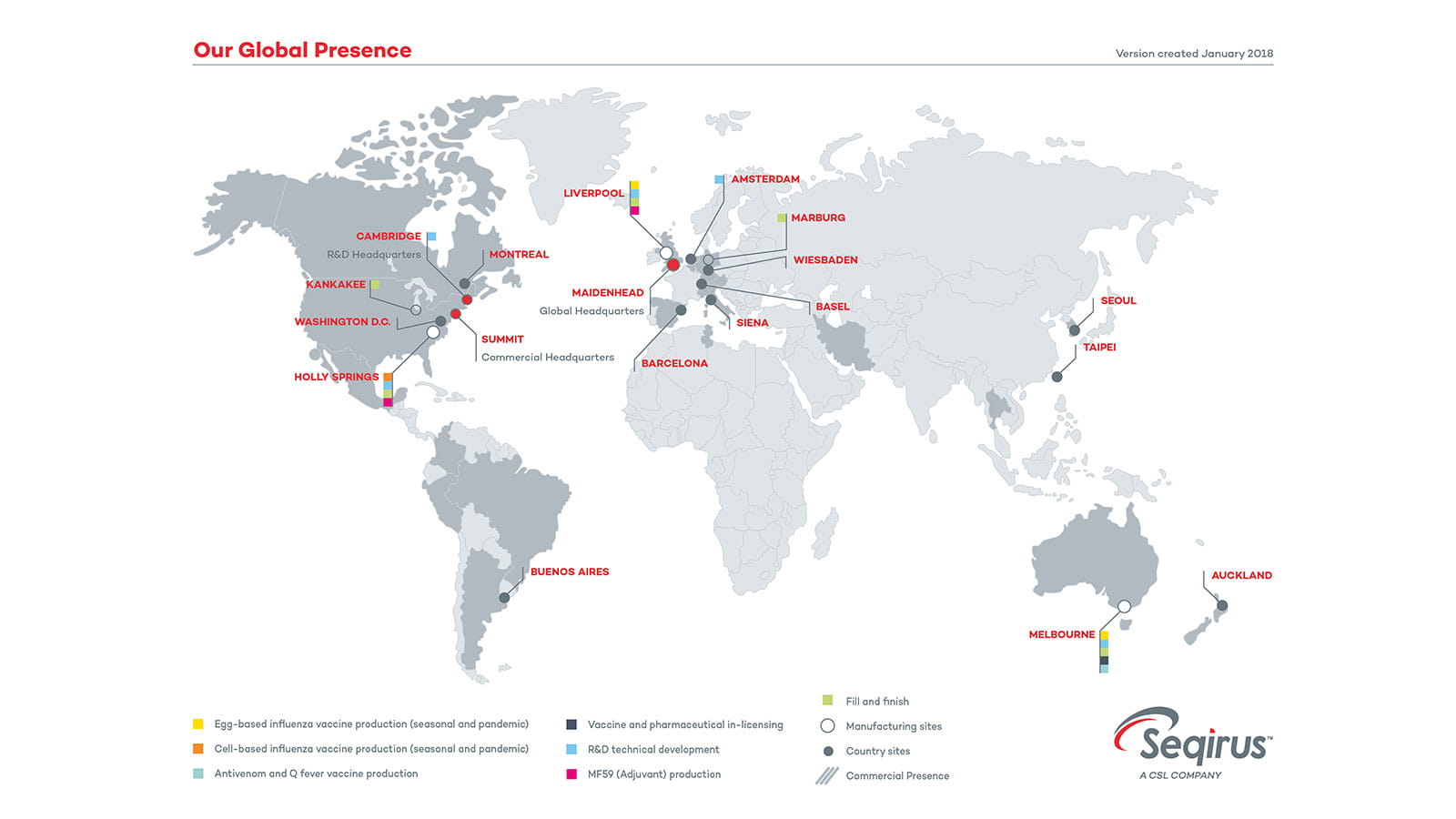
Locations Around the World
CSL Seqirus is the one of the largest influenza vaccine companies in the world, with state-of-the-art manufacturing facilities on three different continents.

CSL Seqirus utilises both egg and cell-based production technologies to produce influenza vaccine. The year-round production of seasonal influenza vaccine for both northern and southern hemispheres, enables us to be in a constant state of readiness to respond to a pandemic emergency.
With extensive research and production expertise and manufacturing plants in the US, UK and Australia, CSL Seqirus is a transcontinental partner in pandemic preparedness and plays a major role in the prevention and control of influenza globally. It’s a role we are proud of and take very seriously.
CSL Seqirus is the one of the largest influenza vaccine companies in the world, with state-of-the-art manufacturing facilities on three different continents.

CSL Seqirus’ manufacturing plants have a deep technical expertise in the science and manufacture of influenza vaccines and produce a broad portfolio of differentiated products.
The only injectable influenza vaccine facility in the UK, the plant utilises egg-based and adjuvant technology for seasonal, pre-pandemic and pandemic vaccines.
The CSL Seqirus Liverpool site is a centre of excellence for the manufacture of enhanced influenza vaccines and plays an important part in providing pandemic preparedness and response for the UK and other countries in Europe. An egg-based bulk manufacturing facility, Liverpool produces seasonal influenza vaccine distributed across the northern and southern hemispheres. With over 650 employees, it is one of the biggest biotechnology sites in Europe.
The largest cell-based influenza vaccine manufacturing facility in the world, with advanced technology successfully producing at commercial scale.
This CSL Seqirus Holly Springs facility was purpose-built in partnership with the U.S. Biomedical Advanced Research and Development Authority (BARDA) to help combat pandemic threats. The public-private partnership is the first in the world to establish cell-based technology as a highly scalable method of production. Additionally, the facility produces the CSL Seqirus proprietary adjuvant, MF59®, which can have a dose-sparing effect, thereby further boosting the output of influenza vaccine during a pandemic emergency. Holly Springs has capacity for formulation, fill and finish manufacturing of seasonal influenza vaccines for global markets.
The only influenza vaccine manufacturing facility in Australia and the world’s only manufacturer of Q fever vaccine and local antivenoms.
Based in Melbourne, Parkville uses egg-based influenza vaccine manufacture for seasonal, pre-pandemic and pandemic vaccine production to Australia and worldwide markets. The facility also plays a key role in the global fight against influenza by developing candidate vaccine viruses for the World Health Organisation in the Asia Pacific region. CSL Seqirus also plays a unique public health role, continuing a long legacy as the manufacturer and sole supplier of a range of products made in the national interest for the Australian Government, including antivenoms and Q fever vaccine.
While CSL Seqirus was established in 2015, we have a rich heritage in the manufacture of influenza vaccines. As part of the CSL Group of companies, our experience dates back to the 1918 deadly influenza pandemic and we’ve worked with global health protectors to combat influenza ever since. From 1942, CSL has been the only onshore producer of influenza vaccine for Australia, and in 2002 we began exporting modest volumes of vaccine to Europe, and later to the US. CSL acquired the Novartis influenza business in 2015 to give its existing influenza vaccine operations global scale, a differentiated product portfolio and greater commercial reach.

CSL Seqirus is committed to bringing innovative technologies for influenza prevention to Australia, such as adjuvanted vaccines and cell-based manufacturing.

Each and every influenza season, we use our deep technical expertise to ensure our technology platforms are performing to their best.
AP-CRP-23-0001


